Alternative Fuels Information Middle
페이지 정보
작성자 Stuart Sears 댓글 0건 조회 2회 작성일 24-11-25 21:06본문
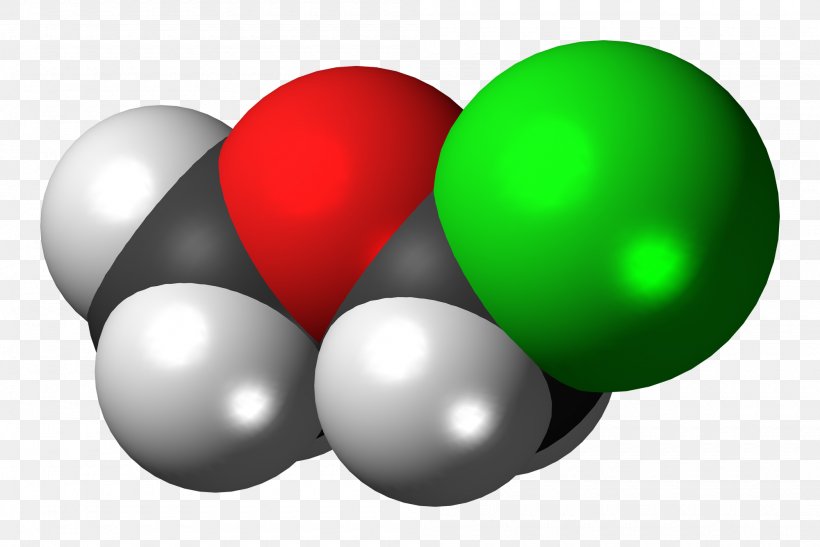
Dimethyl ether (DME) is a synthetically produced various to diesel for use in specially designed compression ignition diesel engines. Below regular atmospheric conditions, DME is a colorless gas. It's used extensively within the chemical business and as an aerosol propellant. Dimethyl ether requires about 75 pounds per sq. inch (psi) of pressure to be in liquid type. Because of this, DME's dealing with necessities are much like these of propane—both must be saved in pressurized storage tanks at an ambient temperature. The results are rapidly reversible which is consistent with the very speedy bioelimination of the molecule. DME has, in the past, been thought-about to be used as a human anesthetic. It must be noted that this chemical can produce cardiac sensitization similar to the consequences of epinephrine. DME released to the ambiance would be expected to exist virtually totally within the vapor phase because the vapor strain is 4450 mmHg at 25 ℃. It is susceptible to photooxidation through vapor phase reaction with photochemically produced hydroxyl radicals. An atmospheric half-life of 5.Four days has been calculated. It may even exhibit very highmobility in soil and, therefore, itmay leach to groundwater.
The company's enterprise operations are organized into three major groups: World Environmental & Infrastructure Enterprise Group, Industrial Finance, Logistics & Improvement Group, and Power Business Group. Royal Dutch Shell, commonly often known as Shell, is a British-Dutch multinational oil and fuel company. The company is organized into four main enterprise segments: Upstream, Downstream, Integrated Gas, and New Energies. The Upstream phase is concerned in exploration and manufacturing activities, including conventional and unconventional oil and gasoline resources. The Downstream section consists of actions equivalent to refining, advertising and marketing, and buying and selling of oil and gasoline products, as nicely because the manufacture and advertising of chemicals. The Integrated Gasoline phase is concerned in the manufacturing and advertising and marketing of liquefied natural gasoline (LNG) and other fuel-associated activities. The new Energies phase is targeted on creating and investing in low-carbon power sources, such as wind and solar power.
Charged species as a rule dissolve readily in water: in other phrases, they are very hydrophilic (water-loving). Biphenyl, like sodium chloride, is a colorless crystalline substance. Biphenyl does not dissolve in any respect in water. It is a really non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. It has some intermolecular forces bonding it to itself via nonpolar London dispersion forces, nevertheless it has no vital attractive interactions with very polar solvent molecules like water. In the meantime the water molecules themselves are highly linked to one another via hydrogen bonding forces. Included within the FDA Inactive Components Database (topical aerosols). Included in nonparenteral medicines licensed within the UK. Included in the Canadian Checklist of Acceptable Non-medicinal Ingredients. Dimethyl ether, also referred to as methoxymethane, wooden ether, dimethyl oxide or methyl ether, is the only ether. Dimethyl ether(DME), also known as methyl ether and methoxymethane, is the simplest fatty ether. Dimethyl ether is a colorless compressed liquefied gasoline or CAS 115-10-6 liquid. 27.0%. Hazard Identification(primarily based on NFPA-704 M Rating System): Health 2,Flammability 4, Reactivity 1. Soluble in water. Dimethyl ether is a liquefied gas and exists as a liquid at room temperature when contained underneath its personal vapor strain, or as a fuel when uncovered to room temperature and stress. It is a transparent, colorless, just about odorless liquid. In high concentrations, the gasoline has a faint ether-like odor.
Exception: Hydrogen tends to attain 2 electrons in its outermost shell to satisfy the He configuration. This is understood because the octet achievement rule. If we examine the weather within the dimethyl ether molecule, we discover that all the hydrogen and carbon atoms have achieved their corresponding required configurations. However, oxygen only has 4 valence electrons surrounding it. We've fulfilled the octet rule and used all of the 20 valence electrons. Moreover, DME is gaining momentum as a substitute transportation fuel for diesel owing to its higher ignition in compressed engines. It is cleaner, possesses propane-like properties, and has the potential to replace diesel in heavy-obligation applications. Strong progress within the sales of heavy trucks and vehicles will accelerate the overall dimethyl ether market progress in the course of the forecast period.
These electrons kind groups ( the lone pairs and the bonded pairs) and we consider the central atom only in VSEPR theory for ease of calculation. So, we have now the AX2E2 notation for the methoxymethane molecule. As we can see, we get a bent molecular geometry like water right here. The bond angle of C-O-C is round one hundred ten levels as a result of methyl groups that experience steric repulsion. The electron geometry is tetrahedral. The top companies within the dimethyl ether market are investing in developing new applied sciences that can improve the properties of DME, thus resulting in the development of recent DME-primarily based products with improved efficiency characteristics. Learn about opportunities, challenges, and traits in the global Dimethyl Ether (DME) market with IMARC’s market analysis report. Dimethyl ether(DME), also called methyl ether and methoxymethane, is the only fatty ether. It's a derivative of the dehydration condensation of two molecules of methanol. It is colorless, non-toxic, gasoline or compressed liquid with slight ether aroma at room temperature. Dimethyl ether has wonderful combustion characteristics, high cetane quantity, low pollution, no damage to the atmospheric ozone layer, and straightforward degradation in the troposphere. It may be widely used as automobile gasoline.
댓글목록
등록된 댓글이 없습니다.